Research Overview
Stress plays a critical role in shaping animal performance, influencing everything from movement and growth to reproduction and survival.
By altering biochemistry and physiology, stressors limit performance. Understanding the mechanisms that underlie susceptibility and tolerance to stressors is essential to predicting how local and global changes will affect animal populations or develop effective strategies for sustainable animal rearing.
In the MacMillan Lab, we study how and why different variables in an insect’s environment, like extreme temperatures, plastic pollution, or diet, set limits to individual performance and survival, and how differences in tolerance to these stressors among individuals, populations, and species arise.
By integrating observations at the molecular, cellular, tissue, organ, and whole animal levels we build and test conceptual models in an effort to explain performance. Using these models as a backbone for new questions, we study the mechanisms that allow for the wide variation in fitness we observe among individuals, populations, or species in nature.
To find information on our research history, see the Publications page.
To find out what we are up to at the moment, read on!
Work In Progress:
The ionoregulatory collapse model of chill tolerance
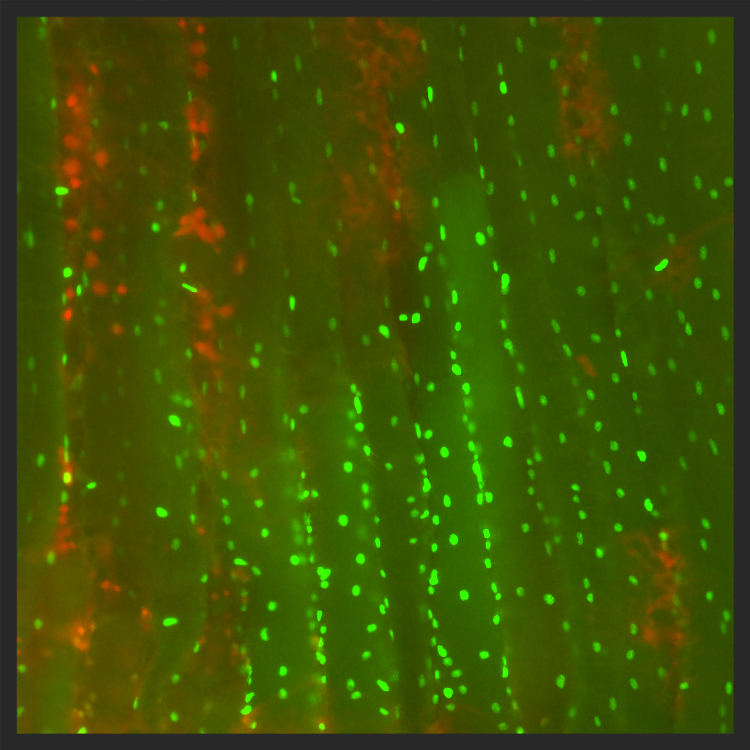
Muscle fibers of a locust after exposure to -2°C for 48 hours. Live cells are green and dead cells are red. Image from MacMillan et al, (2015) Proc. Roy. Soc. B 282: 20151483
Although some insects are adapted for life in the cold, the majority of insects cannot survive exposure to low temperatures, let alone freezing. Like humans, these "chill susceptible" insects are instead injured and killed by relatively mild drops in body temperature.
We use an integrative approach to examine how a loss of ion and water homeostasis leads to a progressive rise in extracellular potassium (hyperkalemia) in a diverse range of animals, including insects and crustaceans. This hyperkalemia depolarizes cells, and is thought to subsequently initiate cell death. We are examining how various aspects of an insects physiological status impact the tendency to drift away from ionoregulatory homeostasis, and how the downstream effects of hyperkalemia drive cellular and whole animal injury.
Example publications:
Davis, H. E., Cheslock, A., & MacMillan, H. A. (2021). Chill coma onset and recovery fail to reveal true variation in thermal performance among populations of Drosophila melanogaster. Scientific Reports, 11(1), 1–10.
El-Saadi, M.I., Ritchie, M.W., Davis, H.E., MacMillan, H.A. Warm periods in repeated cold stresses protect Drosophila against ionoregulatory collapse, chilling injury, and reproductive deficits. Journal of Insect Physiology 123: 104055.
MacMillan, H.A. (2019) Dissecting cause from consequence: A systematic approach to thermal limits (Invited). The Journal of Experimental Biology 222: jeb191593.
The consequences of microplastic ingestion in terrestrial insects
Image credit: Serita Fudlosid
Megatons of plastics are directed to terrestrial ecosystems but the majority of our knowledge on the effects of plastic pollution are focused on marine animals. Through support from Environment and Climate Change Canada, and in collaboration with Dr. Jennifer Provencher, we are exploring the effects of plastic feeding on model insects. This project integrates from the level of plastic effects on insect growth and fitness right down to how feeding on plastic alters gene expression and organ function. We hope to identify the consequences of eating plastic (or lack thereof) to inform future lab practices to identify and describe these effects in a wide variety of terrestrial animals.
Example publication:
Fudlosid, S., Ritchie, M. W., Muzzatti, M. J., Allison, J. E., Provencher, J., & MacMillan, H. A. (2022). Ingestion of microplastic fibres, but not microplastic beads, impacts growth rates in the tropical house cricket Gryllodes sigillatus. Frontiers in Physiology, 0, 725.
Edible insects
Newly emerged Gryllodes sigillatus nymphs, our model organism. Photo: Matt Muzzatti
Although many countries around the world have embraced the idea of eating insects, Europe and North America are only now catching up. Insects have the potential to be used to produce large quantities of protein in an environmentally responsible way, and are considered a superfood of the future. In collaboration with the Bertram Lab and our partners at Entomo Farms, we are working to maximize insect growth and welfare to advance the North American entomophagy industry. Together, we are tackling real farm challenges with the power of science!
The renal and endocrine control of cold tolerance
A suite of recent evidence has allowed us to build the ionoregulatory collapse model of insect chill tolerance (above). The insect renal system is responsible for the maintenance of hemolymph ion homeostasis, and as such it represents both a critical target of cold induced failure and an important potential site of thermal adaptation and plasticity. The insect renal system is also under tight neuroendocrine regulation, and as such insect neuropeptides may be essential in coordinating rapid responses to thermal challenges.
Mainly using Drosophila as a model, we are examining precisely how the various components of the insect renal system are affected by low temperature, whether the endocrine system coordinates thermal plasticity, and how disruption of endocrine signalling can alter cold tolerance at the level of the whole animal.
Example publications:
Jass, A., Yerushalmi, G.Y., Davis, H.E., Donini, A., MacMillan, H.A. (2019) An impressive capacity for cold tolerance plasticity protects against ionoregulatory collapse in the disease vector, Aedes aegypti. The Journal of Experimental Biology 222: jeb214056
MacMillan, H. A., Nazal, B., Wali, S., Yerushalmi, G. Y., Misyura, L., Donini, A. and Paluzzi, J.-P. (2018). Anti-diuretic activity of a CAPA neuropeptide can compromise Drosophila chill tolerance. The Journal of Experimental Biology. 221: jeb.185884.
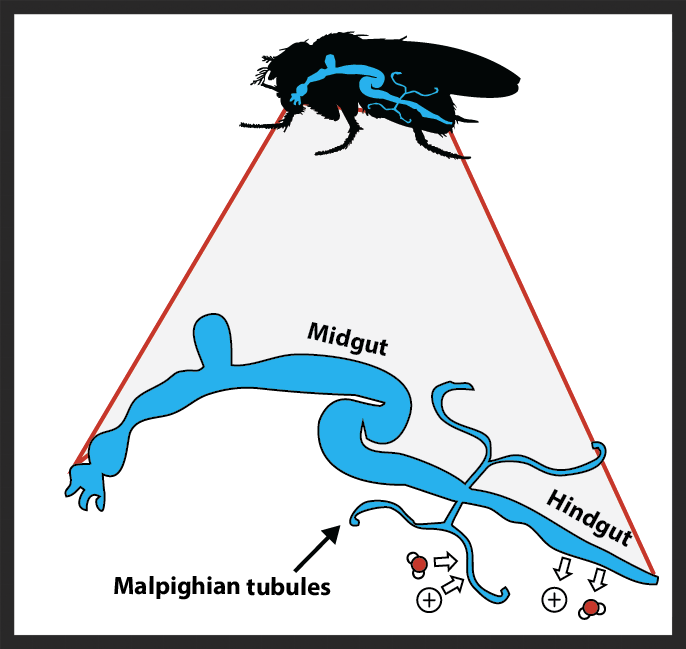
Insect paracellular occluding junctions
The foregut of a transgenic fruit fly with green fluorescent protein bound to a major component of the septate junctions.
The milieu of various body compartments are efficiently separated by paracellular junctions that bind together epithelial cells. These junctions are critical components of the hemolymph-brain and hemolymph-gut barriers in insects, and are thus requisite for survival, but their structure and function are poorly understood in insects relative to vertebrates like ourselves. We are particularly interested in how temperature impacts junctions structure, stability, and permeability and how thermal adaptation and acclimation shape barrier function. Along the way we are learning new and exciting information about the basic biology of these understudied cellular components.
Example publications:
Brzezinski, K., MacMillan, H.A. (2020) Chilling induces unidirectional solute leak through the locust gut epithelia. The Journal of Experimental Biology 223: jeb215475.
Livingston, D.L.,Patel, H., Donini, A., MacMillan, H.A. (2020) Active transport of brilliant blue FCF across the Drosophila midgut and Malpighian tubule epithelia. Comparative Biochemistry and Physiology A: Molecular and Integrative Physiology 239: 110588.
MacMillan H.A., Yerushalmi, G., Jonusaite, S., Kelly, S.P., Donini, A. (2017) Thermal acclimation mitigates cold-induced paracellular leak from the Drosophila gut. Scientific Reports. 7, 8807.
Transcriptional and metabolic responses to temperature change
Cold acclimation can massively reorganize the Drosophila transcriptome. Figure from MacMillan et al. (2016) Scientific Reports 6: 28999.
How do patterns of gene expression change with time spent at low temperatures, and can we use these patterns to better understand how thermal adaptation works? Is there overlap in the genes that show differential expression following cold acclimation and changes in metabolite concentrations observed at the whole animal level? We use modern bioinformatic and biochemical methods to characterize and interpret the massive reorganization of gene expression and metabolic function that occurs upon a change in environmental temperature. At present, we are working to deepen this understanding through a particular focus on the organs and tissues most relevant to chilling tolerance.
Example publication: MacMillan et al., 2016. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep. 6, 28999.
Cross tolerance
In the real world, animals can encounter multiple environmental stressors simultaneously, but our current understanding of stress tolerance is mostly limited to single stressors acting independently over short time scales in the lab. We are interested in how various stressors (both biotic and abiotic) interactively impact tissue and organ physiology, and how these interactions manifest to alter stress tolerance traits at the organismal level. Right now, we are particuarly interested in the impacts of changes to the diet on thermal tolerance, and how hypoxia and desiccation may interact with thermal stress to influence water and ion balance.
Example publication:
Yerushalmi et al., (2016) Chronic dietary salt stress mitigates hyperkalemia and facilitates chill coma recovery in Drosophila melanogaster. Journal of Insect Physiology 95: 89-97.